Learning Objectives
- The Number Of Valence Electrons In Beryllium
- Number Of Valence Electrons In Li
- What Is The Valence Of Beryllium
- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
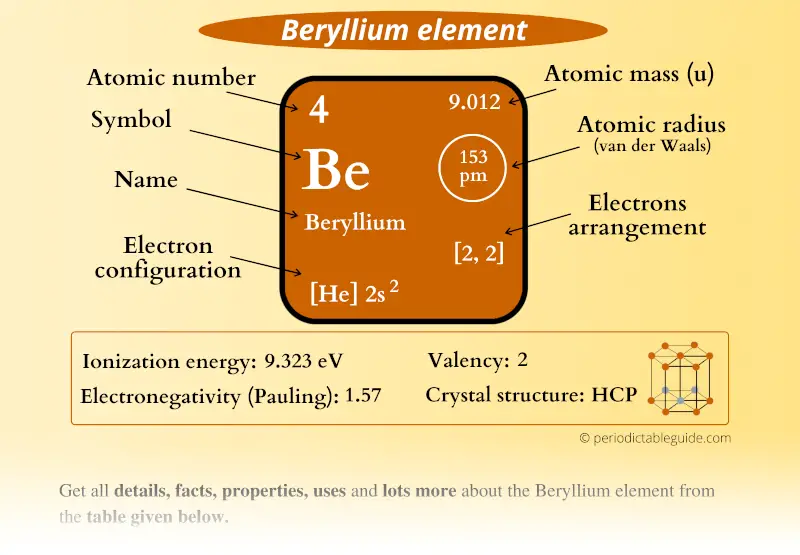
Because beryllium can supply two valence electrons to give oxygen its preferred number of 8 (Figure 15.11). Share 2 electrons. Carbon has four valence electrons. That means two oxygen atoms can bond with a single carbon atom, each oxygen sharing two of carbon’s four valence electrons. There are two ways to find the number of valence electrons in Beryllium (Be). The first is to use the Periodic Table to figure out how many electrons Berylli. Sunday, August 04, 2019 A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Beryllium have? In the case of Beryllium the valence electrons is 2. Element Element Number Number of Electrons in each Level Beryllium 4 2 Boron 5 3 Carbon 6 4 Nitrogen 7 5. What element has a high melting point, is dense, and has two valence electrons? Beryllium The number written to the left of each period on the periodic table represents possessed by elements in.
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
The Number Of Valence Electrons In Beryllium
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
Practice
Use the link below to answer questions about valence electrons:
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
Glossary
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
References
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.
Drawing the Lewis Structure for BeCl2
Viewing Notes:
- The BeCl2 Lewis structure is similar to BeF2 since F is in Group 7 and has 7 valence electrons.
- Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4).
- If you're not sure you have the best Lewis structure for BeCl2 you can calculate the formal charges. You'll find the Be in BeCl2 only has 4 valence electrons.
- For the BeCl2 Lewis structure there are a total of 16 valence electrons available.
See the Big List of Lewis Structures
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for Beryllium Chloride. On the periodic table Beryllium is in group 2, it has 2 valence electrons; Chlorine--group 7--7, but we have 2 of those Chlorines. We multiply that by 2 and add this, we get 16 total valence electrons. Let's draw it. We'll put the least electronegative, Beryllium, at the center, and on either side we'll put a Chlorine. And we have 16 valence electrons. Put 2 between the atoms to form the bonds, and then around the outside atoms, 6, 8, 10, 12, 14, 16.
We've used up all the valence electrons and let's see if we have octets. This is good on this Chlorine, and over here we're fine. We have 8 on both. But the Beryllium in the center only has 4. I know that Beryllium is kind of an exception. It doesn't necessarily need 8 valence electrons.
Number Of Valence Electrons In Li
So I'm not sure if this is the right structure. The way to check is to use formal charges. So we have an equation here to calculate the formal charge for each of the atoms. So let's start with this Chlorine right here. Chlorine's in grou 7, so it has 7 valence elecrons. Nonbonding, these ones right here, there are 6 of those. And bonding, there are 2 of those, but we're going to divide that by 2. Seven minus 6 minus 1 is 0. The formal charge on Chlorine is 0, and since both Chlorines are the same, that's going to be 0, too.
OK, let's check out the Beryllium right here. So Beryllium is in group 2, 2 valence electrons. Nonbonding: well, all of the valence electrons for Beryllium are involved in bonds, so that's 0. Minus bonding, these right here dvided by 2. Two minus 0 minus 2 is 0, so the formal charge on Beryllium is also 0.
What Is The Valence Of Beryllium
When the formal charges are all 0, then I'm comfortable that this is really going to be the best structure. This is Dr. B., and thanks for watching.




